EXXUA™ for Major Depression
Therapeutic Area: Major Depression
- Development Status: FDA Approved.
Product Profile:
- First TRULY NOVEL mechanism for MDD in 30+ years
- “Next Generation” targeted treatment
- Addresses major physician and patient complaints about available therapies –Sexual dysfunction –Weight gain
- Better compliance lowers healthcare burden and costs for payors
- Benefits should justify first line consideration by formularies
- Pricing competitive with other currently available branded antidepressants
- Additional indications and life cycle management strategies
- Peak annual US market sales potential > $1 Billion
- Efficacy in depression comparable to SSRIs and SNRIs.
- Studied in elderly with efficacy and safety similar to younger populations.
- Adverse events in pediatric population mild and similar to adult studies.
- Improvement from baseline in HAMD Anxiety, Psychic Item, versus placebo at all weeks (p<0.050) starting week one.
- Activating and particularly beneficial for patients who demonstrate a lack of energy, psychic and motor retardation, or fatigue.
- Can be co-administered with other antidepressants.
- Can be used to switch patients from SSRIs with no adverse consequences.
- Does not cause sexual dysfunction (equal to placebo).
- Improves sexual function (superior to placebo and SSRIs on validated scales)
- Causes no weight gain (equal to placebo)
- Does not increase suicidal thoughts or impulses in children or adolescents relative to placebo.
- Common adverse events (>10%) of headache, transient dizziness at dose escalations, nausea, and insomnia. Although dizziness is the most common adverse experience reported compared to placebo, the majority of such events were mild to moderate in severity, dose-related, and only 3% led to discontinuation of treatment.
Clinical Trials
2 Pivotal Trials in Depression of EXXUA™ support NDA approval.
The efficacy of EXXUA™ as a treatment for MDD was established in two eight-week randomized, double-blind, placebo-controlled, flexible-dose studies in adult outpatients (age 18 to 69 years) meeting DSM IV criteria for MDD. In both studies,EXXUA™ was superior to placebo as measured by improvement on the 17-item Hamilton Depression Rating Scale (HAMD-17) total score (Table 8).
In one trial, patients received EXXUA™ 20 to 80 mg/day (n=102) or placebo (n=106). After an initial dose of 20 mg/day, patients were titrated to 40 mg/day on Day 4 of treatment. The dose could then be increased to 54.5 mg/day after 7 days, and to 80 mg/day after 14 days. Final mean dose of EXXUA™ was 63.83±13.5 mg/day, and for 66% of patients the final prescribed dose was 80 mg.
In the second trial, patients received EXXUA™ 20 to 80 mg/day (n=124) or placebo (n=124) After an initial dose of 20 mg/day, patients were titrated to 40 mg/day on Days 4 to 7 of treatment. The dose could then be increased to 60 mg/day on Days 8 to 14, and to 80 mg/day after 14 days. The mean dose of EXXUA™ was 60±12.7 mg/day, and for 61% of patients the final prescribed dose was 80 mg.
Table 8.
| Study | Primary Measure | Treatment Group | N | Mean Baseline Score (SD) | Week 8/ETLSMean CFB (SE) | Pbo-subtracted diff (95% CI)* | p-value* |
| 134001 | HAMD-17 | Gepirone-ER | 101 | 22.73 (2.45) | -9.04 (0.78) | -2.47 (-4.41, -0.53) | 0.013 |
| Placebo | 103 | 22.75 (2.51) | -6.75 (0.77) | ||||
| FKGBE007 | HAMD-17 | Gepirone-ER | 116 | 23.9 (2.69) | -10.22 (0.75) | -2.45 (-4.47, -0.43) | 0.018 |
| Placebo | 122 | 24.2 (2.93) | -7.96 (0.73) | ||||
| Baseline on ANOVA with effects for treatment and center; ET=End of treatment; CFB=Change from baseline; SD=Standard Deviation; LS=Least Squares; SE=Standard Error[Source: CSR 134001 Table 12, Appendix F8.6.1.1-2 and CSR FKGBE007 Table 15]
*Difference in baseline-adjusted means from ANCOVA [Source: ISE Tables 3.1 and 3.2] |
|||||||
Time Course of Treatment Response
In the eight-week placebo-controlled studies, an effect of EXXUA™ based on the primary efficacy measure was generally observed starting at Week 2 and increased in subsequent weeks with the full antidepressant effect of EXXUA™ generally not seen until Study Week 3 or later. Figure 1 depicts time course of response based on the primary efficacy measure (HAMD-17) in Study.
Figure 1. Change from Baseline in HAMD-17 Total Score by Treatment Week
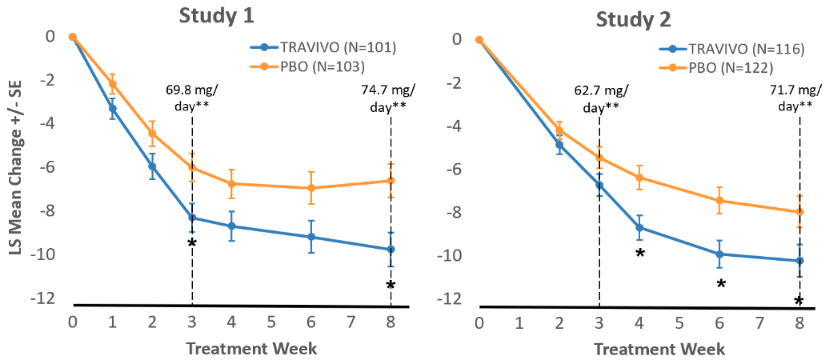
Analyses of the relationship between treatment outcome and age, and treatment outcome and race did not suggest any differential responsiveness on the basis of these patient characteristics.
Maintenance Study
In a longer-term trial, 210 patients meeting DSM-IV criteria for MDD who had responded during an eight- to 12-week open-label treatment phase with EXXUA™ 20 to 80 mg/day were randomized to continue EXXUA™ or to placebo for up to 12 months of observation for relapse. By analysis per protocol, patients receiving EXXUA™ had a statistically significantly lower relapse rate (24.0%) compared to placebo (38.7%). Time to relapse was also statistically significantly longer in patients treated with EXXUA™.
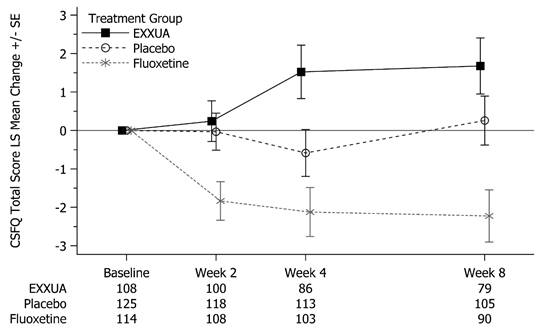
Analysis of data from a group of clinical trials with objective measures of sexual function demonstrates that EXXUA™ has no negative effect on sexual function in patients with MDD. EXXUA™ showed a distinct advantage over SSRIs that are commonly associated with sexual dysfunction.
Sexual Dysfunction
LS Mean Change from Baseline in CSFQ-14 Total Score- Patients with No Sexual Dysfunction at Baseline